Electrical & Magnetic Charges {© 01/06/18}
Electrical and magnetic charges are essential converses, and mutually inclusive; they are equal and opposite, and one cannot exist without the other.
Electrical charges are polar, and shared between all particles.
Magnetic charges are non-polar, and accrue with multiple particles.
Whilst the electrical charge was recognised by Coulomb, and magnetic fields were recognised by Gilbert, it would appear that the magnetic charge has remained undiscovered until now. Magnetic charge is actually what we refer today as 'mass' (or inertia); the resistance to movement of bodies (of mass). Mass (and inertia) is actually the resistance to movement of magnetically charged particles in their universal magnetic environment.
Atomic Particles
There are only two particle types in the universe; the electron and the proton (see neutron):
Electrons carry a negative electrical charge; e⁻.
Protons carry a positive electrical charge; e⁺.
Both electrons and protons carry a magnetic charge; mₑ & mₚ respectively.
Lone Particles (independent)
The magnitude of electrical charge in both particles is equal and opposite; ±e.
The magnitude of an electron’s [non-polar] magnetic charge is; mₑ = |e|.φ
The magnitude of a proton’s [non-polar] magnetic charge is; mₚ = mₑ.ξₘ.
Partnered Particles (proton-electron pair)
There are more than 2.80059013353655E+75 proton-electron pairs in the universe (>4.68687882273808E+48 kg).
The electrical charge held by the proton varies proportionally with its electron partner's orbital energy (temperature; Ṯ); eꞌ = mₚ.RC . Ṯ/Ṯₙ.
The maximum (limiting) electrical charge that can be held by a proton is; eₙ = mₚ.RC.
Note: The temperature of any proton-electron pair is calculated thus: Ṯ = PE/KBꞌ
Where; KBꞌ = Y.KB and ‘PE’ is the potential energy between a proton and its orbiting electron partner.
The temperature we measure in matter is that of the highest energy proton-electron pairs in its atoms; shell 1: Ṯ₁ = PE₁/KBꞌ
The neutronic temperature - the highest possible in nature; that in the core of a bright star - is calculated thus: Ṯₙ = PEₙ/KBꞌ
Their magnetic charges do not vary with temperature.
Electrical and Magnetic Forces
Because magnetic charges accrue, magnetic force (between particles) varies proportionally with the number of particles involved.
Because electrical charges are shared, electrical force (between particles) varies inversely with the number of particles involved.
This is why the gravitational (magnetic) force between celestial bodies, that comprise massive quantities of particles, is significant, whereas the electrical force between them is negligible.
Magnetic charge force is calculated thus: Fₘ = G.m₁.m₂/R²
Electrical charge force is calculated thus: Fₑ = k.e₁.e₂/R²
Due to electrical charge sharing, the electrical charges in a proton-electron pair must be equal to the lowest of the two values (e₁ & e₂); Fₑ = k.e²/R²
where the lowest electrical charge is that of the electron (e)
The remaining electrical charge (eꞌ-e) forces two adjacent atoms apart. As the temperature of adjacent atoms rises, the kinetic energy of their electrons increases, increasing the electrical charge in the protons (eꞌ). When ‘eꞌ-e’ is greater than the magnetic field force holding them together, the atoms will exist as a gas. Conversely, when the magnetic field force is greater, the atoms will exist in a viscous state.
The ratio of magnetic attractive force to electrical attraction/repulsion force in a proton-electron pair is calculated thus:
φ = (G.mₚ.mₑ) / (k.e²) = 4.40742111792334E-40
This means that the electrical-charge repulsion between adjacent atoms in outer-space is 2.27E+39 times greater than their magnetic-charge attraction (gravitational). This is how we know that universal matter cannot possibly accrete from hydrogen atoms.
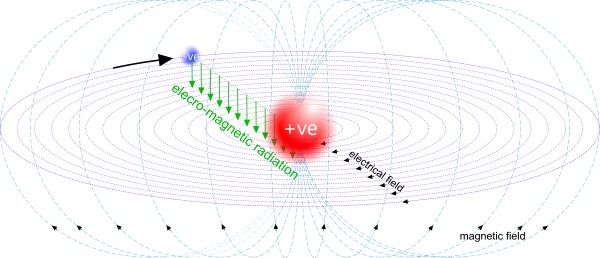
Fig 1. Electro-Magnetic Fields
Electrical and Magnetic Fields
An electro-magnetic charge orbiting another electro-magnetic charge of opposite electrical polarity will generate electro-magnetic fields (Fig 1), that together will emit EME, the polarity of which will vary between e⁻ & e⁺ & e⁻., and also between m⁻ & m⁺ & m⁻ through a single orbital cycle (Fig 2).
The magnetic field generated by the proton-electron charges are responsible for holding onto one or two neutrons (deuterium and tritium respectively).
If you force the proton and its neutron partner(s) of a proton-electron pair inside the orbital shell of another proton-electron partner, you create a different atom. But each proton, electron and neutron partnership remains in-tact - even inside an atom - due to their electrical and magnetic charges.
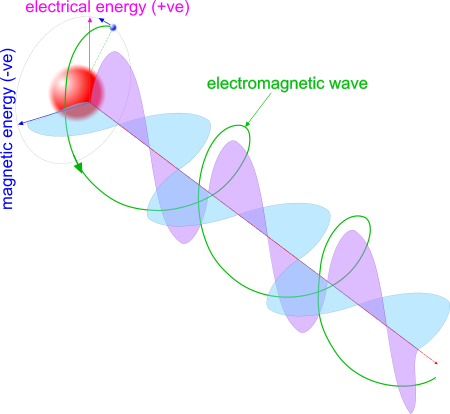
Fig 2. Electro-Magnetic Energy
Universal Forces & Energies
Every force in the universe is generated by the attraction or repulsion between the electrical charges held, and fields generated, by every atomic particle; protons and electrons.
A force multiplied by the distance over which it is applied, is the energy in a body or system.
These charges are responsible for all universal - energy and forces:
kinetic,
potential (gravitational),
electrical,
magnetic,
electro-magnetic (ultra-violet, infra-red, heat, light, gamma, X-ray, micro and radio).
Calculations
Electricity - in all its forms - is directly dependent upon the electrical charge in an electron; the elementary charge unit (e), making the calculation of electrical energy and force not only simple, but also mathematically relatable to any and all other forms of energy (and force).
The Weber is the unit of measurement we use today to define magnetism. But it is an unusable unit of convenience, in that it cannot be related directly to its electrical counterpart (current), and it cannot be explained in terms of the primary constants. This is because we do not understand magnetism as well as we understand electricity.
Today we use the term mass (kilogram) to define the magnetic properties of matter. Mass is not a scientific term; it is another property of convenience that was used to explain something we did not understand. Mass is actually inertia, a body's resistance to movement, which is universally consistent. Whereas inertia is actually the reciprocity between the magnetic charge in a body's atomic particles and the magnetic field radiated by all the atomic particles in the universe; it is the source of what we today refer to as gravity.
If we want to calculate magnetic energy and force in exactly the same way as we do for electricity, we need a magnetic equivalent to the Coulomb, to replace the unit of mass.
The Coulomb is used to describe electrical charge; its behaviour is responsible for all electrical properties; DC and AC.
Looking at the emf-mmf Table, we see that flux is the magnetic equivalent of electrical current; Φ ≡ I
I = Ampere = C/s
so
Φ = Weber = ?/s
but what is ?
Let's refer to mass as 'magnetic charge' and give it the unit 'Gilbert' (G) in deference to William Gilbert.
Φ = Weber.seconds = G
We now know that the coupling ratio defines the ratio of magnetic force to electrical force:
φ = G.mₑ.mₚ / k.e² = 4.40742111792334E-40
so, if we replace mass (m) with magnetic charge (m) and give it the unit Gilbert, we now have magnetic and electrical equivalents.
current: Ampere = C/s
and
magnetic flux: Weber = G/s
The electrical charge in an electron; e = 1/C = 1 / 6.24150964505573E+18 = 1.60217648753E-19 Coulombs
The mass of an electron; mₑ = 9.1093897E-31 kg
If we set the magnetic charge thus; m = e.φ = 7.06146648577996E-59 G
The conversion factor for kg to G; γ = m/mₑ = 7.7518546449E-29 G/kg
Newton's gravitational constant in terms of kilograms;
G = aₒ.c² / mᵤ = 6.67359232004333E-11 {m³ / s².kg}
becomes;
Newton's gravitational constant in terms of Gilberts;
M = aₒ.c² / (mᵤ.γ²) 1.11057637704762E+46 {m³ / s².G}
Now let's check:
the apogee distance between the sun and the earth; R = 1.52094196155084E+11 m
our sun's magnetic charge (mass); m₁ = 1.9885E+30 kg x γ = 154.14562961 G
our earth's magnetic charge (mass); m₂ = 5.964519764E+24 kg x γ = 4.623609024E-04 G
The potential force between the sun and the earth @ R is therefore;
F = M.m₁.m₂/R² = 3.421649076E+22 N
So we now have an equivalent unit of measurement for the magnetic charge in matter and we can use it to calculate magnetic force in exactly the same way as we do with electricity; based upon the primary constants, one of which, 'kilogram' can now be replaced with the 'Gilbert'. We no longer need the units; Siemen, Weber, Maxwell, Henry, etc. none of which allow us to integrate magnetism into mathematical chemistry.
Conclusion
Every form of universal energy and related force - from atomic to celestial - has been successfully calculated using the above electrical and magnetic particle charges.
This theory, therefore, is not hypothesis, it is indisputable fact.
An alternative approach would be to modify Coulomb's constant (k) to reflect Newton's constant 'G', and to use mass in Coulomb's force formula, which is more applicable for the force unit; 'Newton'.
Further Reading
You will find further reading on this subject in reference publications(68, 69, & 70)