The Battery
Battery driven vehicles are becoming compulsory very soon, but how good are they when compared to petrol-driven vehicles?
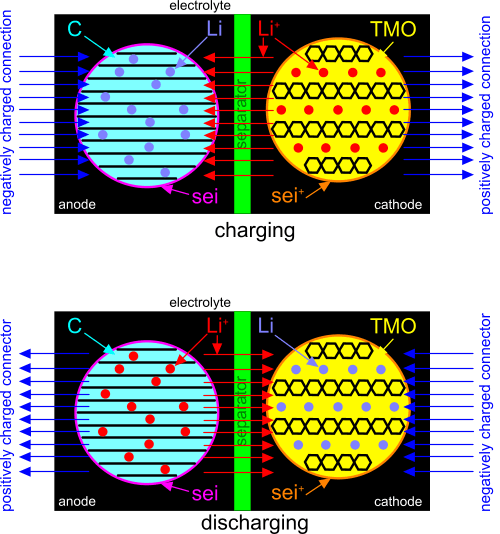
Fig 1. Lithium Ion Battery
Definitions
The following are CalQlata’s definitions for a typical lithium-ion battery (Fig 1).
anode: negatively charged electrode (lithium alloy (Lᵢ.Sᵢ or Lᵢ.C₆))
cathode: positively charged electrode (lithium cobalt oxide (Lᵢ.Cₒ.O₂))
electrolyte; Lithium hexafluorophosphate (Lᵢ.PF₆)
electrolyte shield; solid electrolyte interphase (sei)
positive electrolyte shield; positive solid electrolyte interphase (sei⁺)
transition metal oxides (TMO)
positive lithium ions (Lᵢ⁺)
graphite (C)
separator; permeable membrane (manganese dioxide (Mₙ.O₂))
Note: the separator only allows the flow of positive lithium ions. It does not allow the flow of electrons.
Battery Charging
Connect one end of a charging device to the anode of the battery, the other end to the cathode and switch it on (activate). The process goes something like this:
1) The charging device pulls electrons from the lithium atoms in the cathode making them positively charged; positive ions.
2) These electrons are dragged by the charging device through the activated circuit and pushed into the anode, making it negatively charged.
3) The electrons in the anode cannot pass through the separator, so they pull the positively charged ions through the electrolyte into the anode intercalating them within the graphite lattice structure neutralising the electrons and the ions in the anode.
4) This process continues until the graphite can accommodate no more ions; it is 'full'. When this happens, and the flow of positive ions has ceased, the battery is fully charged.
Discharging
The discharging cycle is almost identical to the charging cycle, but the process is reversed.
Connect the negative end of an external circuit to the cathode of the battery, the positive end to the anode and switch it on (activate). The process goes something like this:
1) The electrical circuit pulls electrons (via a conductor) from the lithium atoms in the anode creating positive ions from its lithium atoms. Some electrons are normally lost to the circuit in this process.
2) These electrons flow through the circuit to the cathode, making it negatively charged.
3) The electrons in the cathode cannot pass through the separator, so they pull the positively charged ions through the electrolyte into the cathode neutralising the electrons and the ions in the cathode.
4) When the flow of ions from the anode to the cathode ceases; (emptying the graphite of lithium atoms), the battery is fully discharged.
Relative Efficiency
Petrol possesses 4.8E+07 J/kg of releasable energy.
There are 8.60882E+25 atoms in one kg of lithium.
The kinetic energy in the outer-shell electron of a lithium atom at 300K is 9.85107E-21 J.
Giving a total energy capacity for lithium at room-temperature of 8.48062E+05 J/kg (Table 1).
But because a lithium-ion battery contains only 12% (per unit mass) lithium, a lithium-ion battery can hold no more than 101767.44 J/kg.
And because you need to input potential energy (Voltage) to release kinetic energy (current), which applies to both charging and discharging, electrical [operational] energy release of a lithium-ion battery is only 25% efficient, giving it an effective energy capacity of 25441.8467005455 J/kg.
Added to which, we must also take into account the efficiencies of the driven system. At best, a battery driven motor (for example), cannot be greater than 0.04% efficient when compared with a petrol-driven motor.
When compared with petrol-power, battery efficiency is; ξ = 2.54418467005455E+04 ÷ 4.8E+07 = 0.053003847% (or petrol is 188,665.55% more efficient than an equivalent battery pack).
However, this result is only valid if 100% of all the lithium in a battery is mobile, which is not the case. To this must be added losses due to the electrical circuitry and the driven system.
In addition to the above, battery driven vehicles are at least 30% heavier than equivalent petrol driven vehicles, reducing their efficiency still further:
KE = ½.m.v²
(KEb - KEp) ÷ KEp = 0.302362205 (Ford Focus)
ξp = 1886.6555 ÷ 0.302362205 = 6239.720013
making the petrol driven vehicle 623,972% more efficient than the battery driven vehicle.
Energy Cost
Next we must compare the cost of energy production. For this exercise we will look at the cost of domestic supply in the UK:
Petrol is charged £1.50 per litre at the pump, but 60% of this is excess tax (over and above normal national taxation), which must be ignored as it has nothing to do with the cost of production.
Electricity sells at 24.5p/kWh, the tax on which is in accordance with the national average.
Ignoring the excess tax on UK petrol, a comparison of cost to the consumer will give us a good indication of the relative cost of energy production.
Petrol: cost = 0.4 . £1.5 /SG /4.8E+07 = £1.56250E-08 per Joule
electricity: cost = £0.245/1000/3600 = £6.80556E-08 per Joule
making electricity 4.36 times more expensive to generate than it costs to recover and refine petrol.
Transport Cost
Next we must consider container costs. A battery pack is no more than the electrical equivalent of a petrol tank.
A replacement fuel tank for a Ford Fiesta costs ≈£125
carrying capacity 42 litres, which = 42 . 0.8 . 4.8E+07 = 1.6128E+09 Joules
the carrying capacity cost = 125 / 4.8E+07 = 7.7505E-08 £/Joule
a modern petrol tank will last 20 years, so the overall cost will be 4.96E-08 / 20 = 3.87525E-09 £/J/yr
A replacement battery pack for an electric Ford Fiesta costs ≈£87/kWh
carrying capacity = £87 / 1000 / 3600 Joules = 2.41667E-05 £/Joule
a battery pack will last anything between 1 and 3 years at maximum carrying capacity; cost = 8.05556E-06 £/J/yr
Making the battery pack ≈2078.721147 times more expensive than a petrol tank.
For Example ...
Irrespective of the energy source (fuel) used to power a given system, the quantity required will always be the same. The only differences - between energy sources - will be their cost and efficiency.
Let's compare petrol and electricity using a typical domestic example to find the difference in cost between battery power and that from petrol; boiling a litre of pure water.
Pure water = H₂O:
one oxygen atom + 2 protons
the oxygen atom comprises 8 proton-electron pairs
the proton has no orbiting electrons
therefore, the only kinetic energy in the water molecule is in the oxygen atom.
The mass of one water molecule is; m = 5.68749E-26 kg;
1kg of water possesses; Nᵒ = 1/m = 1.75825E+25 molecules.
1 H₂O molecule holds:
@292.25K; E₁ = 7.99716E-20 Joules per molecule
@373.15K; E₂ = 1.02109E-19 Joules per molecule
1-litre of water holds:
@292.25K; Eₜ₁ = Nᵒ.E₁ = 1.40610E+06 Joules
@373.15K; Eₜ₂ = Nᵒ.E₂ = 1.79533E+06 Joules
δE = Eₜ₂-Eₜ₁ = 1.79533E+06 - 1.40610E+06 = 389230 Joules
The Cookworks 8325 kettle:
Model KE01301YA-CE
220-240V~50/60Hz
2520-3000W
took 2-minutes 24-seconds to boil 1 litre of water.
P = 389230 ÷ 144 = 2702.986111 Joules per second (Watts)
which is virtually mid-way between the max & min designation for the kettle, validating - yet again - the Newton-Coulomb atom.
mₚ = 389230 ÷ 48,000,000 J/kg = 0.008108958 kg (÷ 0.8 = 0.010136198 litres); @ £1.50p per litre, this will cost 1½p
whereas, because electricity costs 4.36 times more to generate and transmit than petrol, this energy source would cost 7p
But if we were using the electricity from a battery, the cost would be; 2078.721147 x 0.066290734 = 138p
Battery Problems
Due to battery cost and the pollution they create, it is essential to prolong their life, which is already considerably less than that of a petrol tank.
Maximising battery life relies almost entirely on ensuring ion mobility does not exceed that of the separator and electrolyte shields, as excessive charging rates will result in premature damage to both, dramatically reducing the battery's useful life.
The potential damage is listed below:
electrode cracking; reducing ion mobility,
shield disruption; reducing ion mobility,
dendrite generation; shorting the battery,
island generation; reducing effective battery capacity,
electrode plating; reducing ion mobility and battery capacity.
memory loss; reducing battery capacity.
This is why you must always fully drain and fully charge a battery, and never charge it faster than its ion mobility allows.
In addition to the above, one must take into consideration the fact that batteries lose power with reducing temperature. For example; a lithium-ion battery will lose 10% of its energy capacity from 23°C to 0°C.
A runaway battery fire is virtually impossible to extinguish, making it far more dangerous than a petrol fire, which can be put out with a conventional fire extinguisher.
Conclusion
Even if an electrical motor is 100% efficient (impossible), and a conservative 33% is applied to internal combustion engines; (ξ = ξp/3 / ξb/1)
the energy in petrol is more than 207,990.67% more efficient, 4.36 times cheaper to generate, and 2,079 times less expensive to convey than in a battery.
Added to which is the cost of recycling: a typical metal petrol tank costs between £4 and £9 to remelt, whilst a small (Ford Focus) battery pack costs about £1,000, making it 200 times more expensive.
And we shouldn't forget that batteries are far more dangerous than petrol, and transport technology is today quite rightly focusing on reduced weight.
And given the inconvenience of rechargeable batteries, together with the pollution they generate, it would appear that the internal combustion engine scores heavily over every aspect of the battery driven vehicle.
When considered together with the difficulty every country is having with electrical energy generation, battery-powered vehicles don't look too promising; do they!
It is extremely difficult, therefore, to justify the use of a battery-powered vehicle over the internal combustion engine, let alone impose it on a nation as a matter of decree.
It makes us wonder; how much due dilligence (if any) do our governments carry out before making decisions that affect their national economy, or is their something else going on behind our backs?
Energy Source
An additional consideration when comparing petrol and battery powered vehicles is the relative efficiency of their fuel source …
Energy is extracted directly from petrol; there is no need to convert it.
This process has a single level of inefficiency.
Electricity is generated from a fuel source#, transmitted to a charger##, stored in a battery### and transmitted## to an electric motor.
This process has four levels of inefficiency.
# hydro-electric power stations are extremely inefficient and damaging to our natural environment.
## electrical transmission always results in significant losses, petrol transmission does not.
### battery operational life and efficiency are both seriously compromised through fast-charging and unconditioning.
In other words; even before comparing their relative operational efficiencies (see above), petrol driven vehicles are already considerably more than four times more efficient than battery driven vehicles because their 'fuel-to-energy' process results in no conversion or transmission losses.
Newton-Coulomb Atom Verification
'thundersaidenergy' claims that lithium has an energy capacity of between 200 and 300 W.h/kg {J/s . hr/kg}, so let's assume 250;
Eₜ = 250 x 3600 = 9E+05 J/kg
The ionisation energy in a lithium atom at 300K according to Newton-Coulomb is calculated as follows:
The temperature of the proton-electron pair (Ṯ̱ₛ), the electron of which is orbiting in lithium's outer shell (shell-2; N=2);
Ṯ̱ₛ = Ṯ/N = 150 K
The orbital velocity of the electron in lithium's outer shell (according to Newton-Coulomb) is calculated thus;
v² = Ṯₛ/X (m/s)²
Its kinetic energy is therefore;
KE = Nₑ . ½.mₑ.Ṯₛ/X J
where Nₑ is the number of electrons in the outer shell (1 or 2). In the lithium atom; Nₑ = 1
KE = 1 . ½.mₑ.150/X = 9.85107E-21 J
The ionisation energy capacity of lithium is therefore;
Eₙ = KE/mₐ = 848061.5567 J/kg
where; mₐ is the atomic mass (1.161598961557E-26 kg)
The ratio of the 'thundersaidenergy' estimate and Newton-Coulomb's calculation is ...
Eₜ:Eₙ = 9E+05/848061.5567 = 1.061243719
... yet further vindication of the Newton-Coulomb atom.
Further Reading
You will find further reading on this subject in various specialist locations, but CalQlata suggests Stanford University's; Oliver Friedman for its operational aspects.