Documented Properties of Elemental Matter
Densities of the Elements
This Table is a collection of the densities of viscous elemental matter (@ 273 K) according to the sources listed below:
Fig 1 shows a good (reliable) match between the selected documented values for viscous matter (@ 273K):
The gaps in Fig 1 are for those elements that exist as a gas at 273K and ambient pressure. CalQlata lacks confidence in published values for these elements in a solid state.
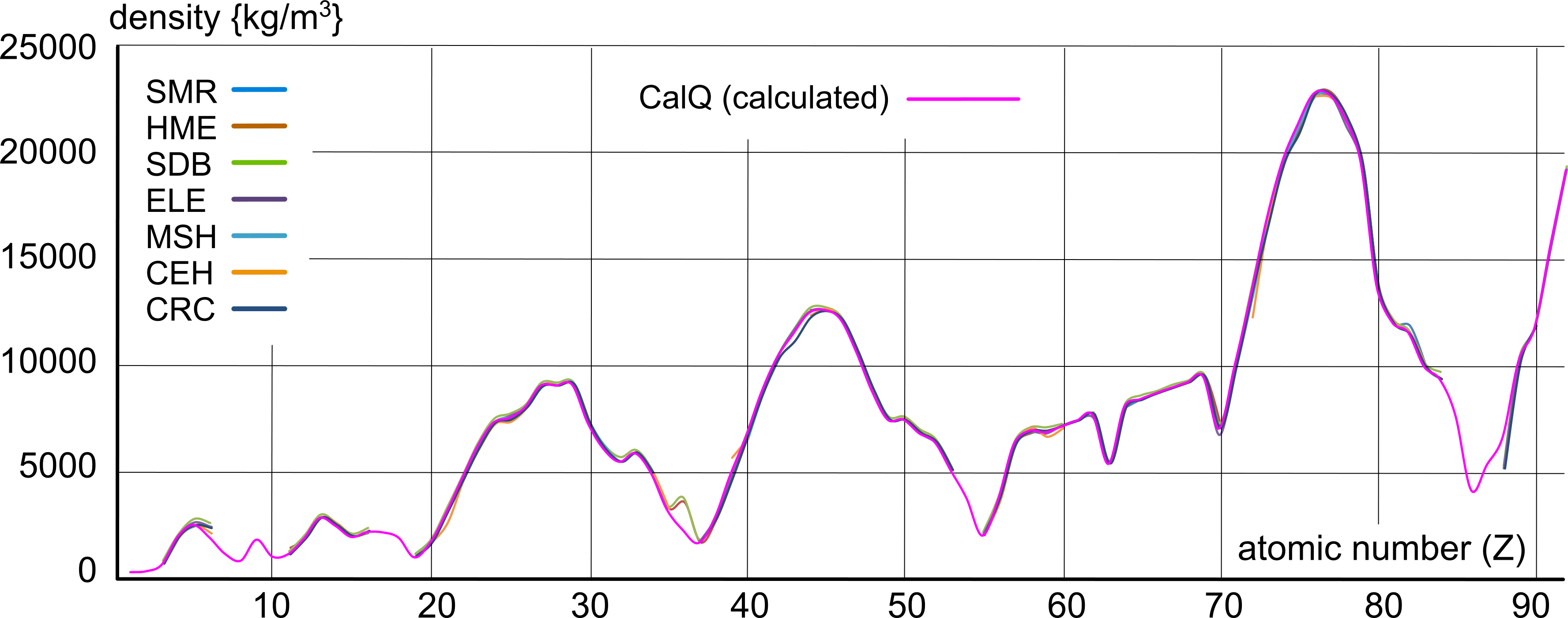
The density of matter may be found using the PVRT formula, which has been well recognised and accepted universally for decades, and which works for all matter in both gaseous and viscous states. It is defined in elemental matter by its atomic mass (mₐ) and the average distance between them (d). Therefore, once we find 'd' we can use it to define the density of matter; ρ = mₐ/d³
p.V = Rᵢ.n.Ṯ {J/K/mole . mole . K = J}
n = mₐ ÷ (RAM/1000) {kg / kg}
p = Rᵢ.mₐ.Ṯ / V.(RAM/1000) {J/K/mole . kg . K / (d³.kg/mole) = kg.m²/s² / m³ = kg.m/s² / m² = N/m²}
V = Rᵢ.mₐ.Ṯ / p.(RAM/1000) {N.m/K/mole . kg . K / (N/m²) (kg/mole) = m³}
ρ = mₐ/V
d = ³√V
The resultant densities are listed in the above Table under the heading CalQ, and plotted in Fig 1, along with the documented values, where it can be seen that both the documented and calculated values are consistent.
Gas Transition Temperature of the Elements
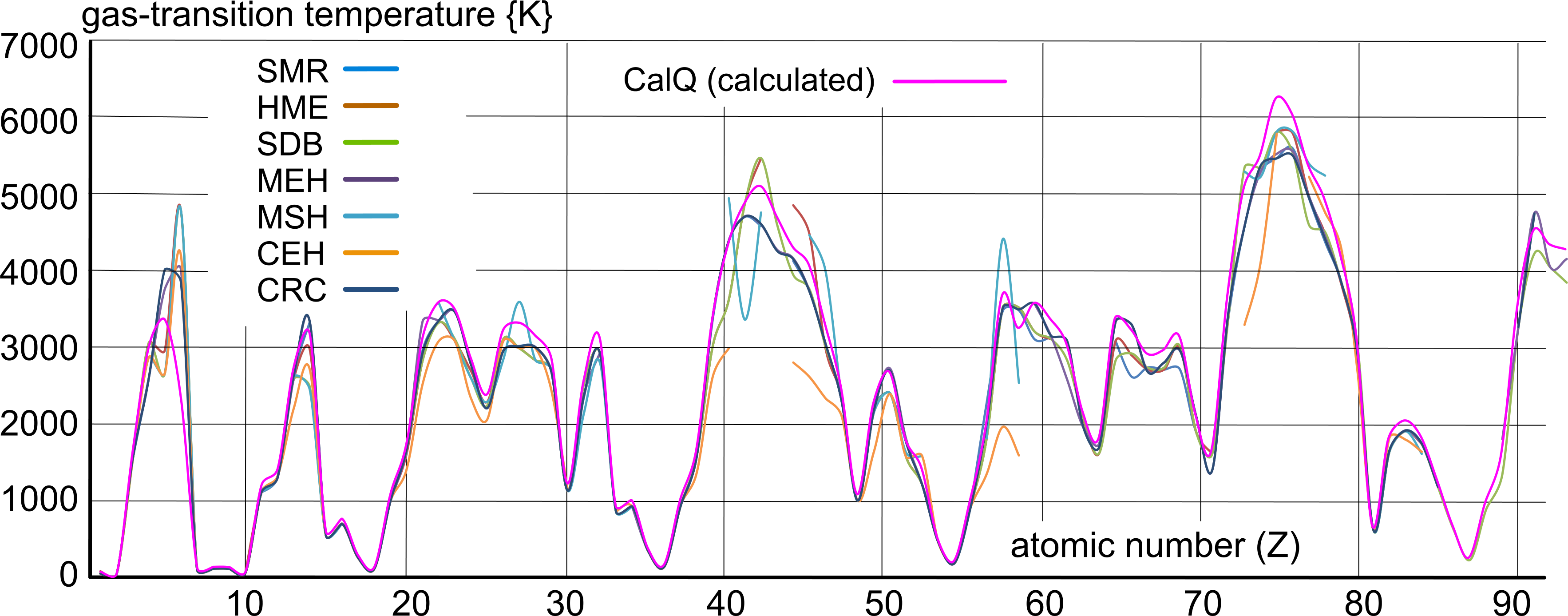
This Table is a collection of the boiling points of elemental matter according to the sources listed below:
Fig 2 shows a poor (unreliable) match between the selected documented values; but it does reveal a recognisable pattern that follows elemental density, and also appears to reflect the calculated values.
Caution
The empirical measurements provided in the above Tables have been taken from numerous sources, all of which are claimed to be as a result of laboratory measurements, which are of course, subject to experimental error, such as;
1) Measurements taken using equipment that is not manufactured from suitable materials.
2) Experiments not usually taken on a pure crystal of the subject material.
3) Experiments not necessarily carried out in a vacuum; e.g. no consideration is given to atmospheric pressure, which will affect theoretical (accurate) values for boiling temperature.
4) Whilst most documented values vary with each other, those provided on internet sites are simply copied with no form of verification.
5) Documented values such as those highlighted in red⁽⁴⁾ are at best questionable.
6) Values for carbon, for example, are not always specified according to form; diamond, graphite, coke, etc.
7) The density of metals such as mercury that have been measured @ 300K in liquid form, meaning that its volumetric state will have given unrepresentative values.
8) The rare earth elements are of particular concern due to; a) the limited work performed on pure crystals of these elements due to the expense, and b) their variability.
The term "N/A" in the above Tables applies to both 'not applicable' for gaseous matter at 273K and also 'not available' where documented data is unavailable in the sources concerned.
The term 'caution' is applied to data deemed unreliable by CalQlata, for example the boiling point of Lanthanum; when five of the sources quote a value close to 3700K, the other two - neither of which supports the other - 2000K and 4700K must be deemed unreliable.
Sources
The following is a list of the documentation sources from which the above data has been selected.
CalQ: Actual (calculated) values based upon inter-atomic forces: Ṯ₁ = hₑ².mₚ / Y.kB . (ψ.ζ)³/d²
SMR: Smithell's Metals Reference Book; E A Brandes & G A Brook; Butterworth
HME: Handbook of Metal Etchants; Perrin Walker, William H. Tarn; CRC Press
SDB: Science Data Book; R M Tennent; Open University
MEH: Mechanical Engineer's Handbook; Myer Kutz; Wiley
ELE: The Elements; Theodore Gray & Nick Mann; Black Dog & Leventhal Publishers Inc.
MSH: Mark's Standard Handbook for Mechanical Engineers; Theodore Baumeister; McGraw Hill
CEH: Chemical Engineer's Handbook; Robert H Perry & Cecil H Chiltern; McGraw Hill
CRC: Handbook of Chemistry and Physics; W M Haynes; CRC Press
Comments: CalQlata believes (rightly or wrongly) data provided in 'published books' may be more reliable than website sources, simply because, CalQlata also believes that greater care is taken over data verification.
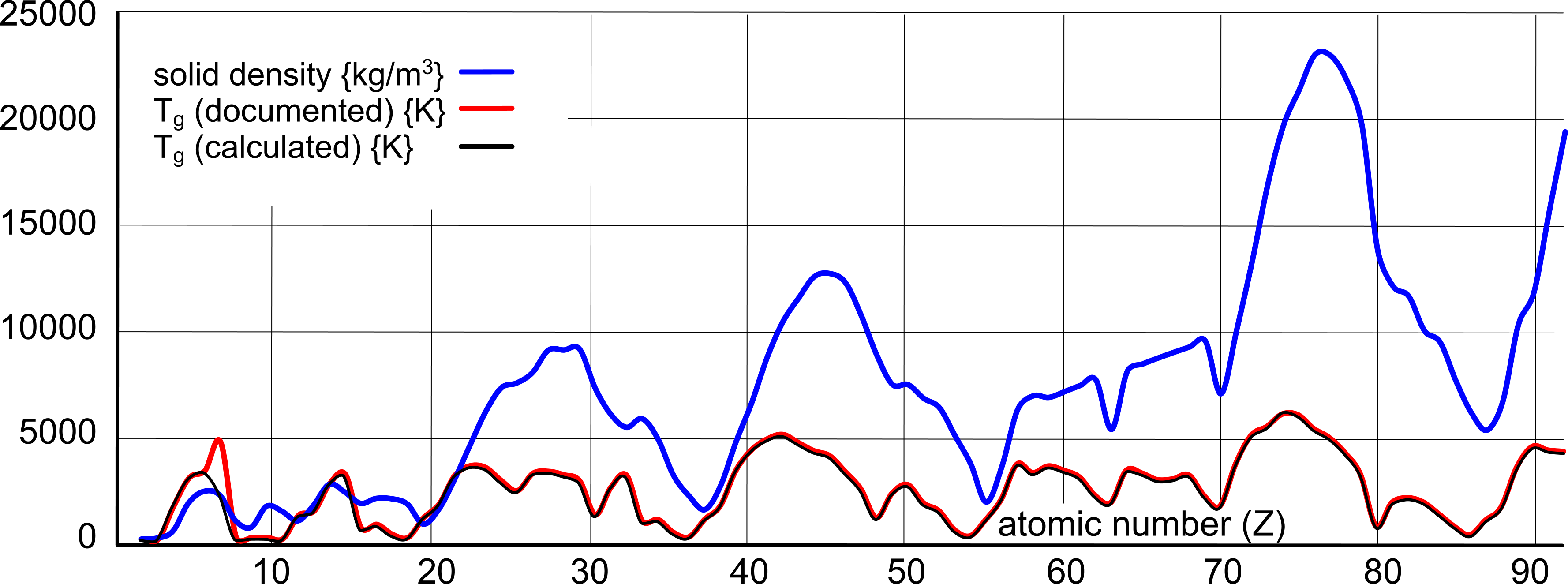
Fig 3 shows the relationship between gas-transition temperature and density of all the natural elements:
Notes
- the values for carbon are for amorphous matter. Diamond and graphite values are quoted thus: diamond density 3512 kg/m³ & boiling point 2273 K; graphite density 2260 kg/m³ & boiling point 5103 K
- Liquid densities of elements that are gaseous at 273.15 K.
- 'Best-fit' estimate densities of elements that are currently unknown Fig 3.
- comment 'caution' is applied to elements the values of which appear unreliable to CalQlata; they may not, however, actually be incorrect.