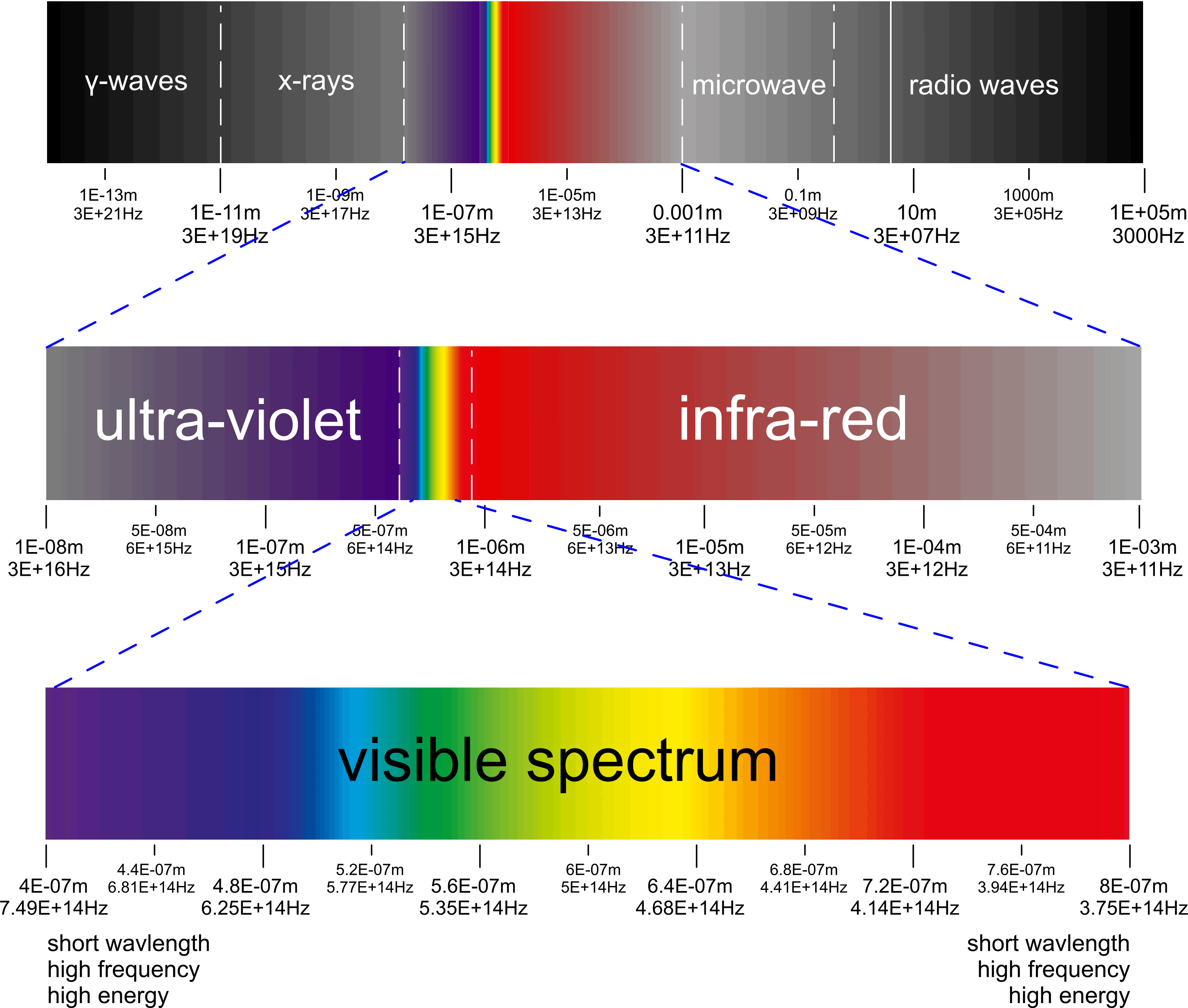
Electro-Magnetic Spectrum
Electro-magnetic energy (EME) is emitted by proton-electron pairs.
Its wavelength (e.g. colour) is defined by its energy, and its brightness is defined by its intensity.
EME is an helical emanation that always travels at the same velocity - irrespective of its energy - that which has come to be known as; the speed of light (c)
The Spectra
You will see many limits related to the various spectral identities (visible light, microwave, etc.) but there is no discrete demarcation for any of them. Like most organic ranges, the lines dividing the various ranges are approximate only; there is considerable overlap.
The mathematical relationship between wavelength (λ) and frequency (ƒ) of electro-magnetic energy is as defined thus: c = λ.ƒ
and the relationship between EME energy and c is defined thus; c = √[ E/mₑ ] (Henri Poincaré)
where E = kB.Y.Ṯ
Because electro-magnetic radiation is only very slightly influenced by gravity (refer to Relativity is Dead), all of it travels in [virtually] straight lines, hence the need for high terrestrial aerials and orbiting satellites for atmospheric transmission.
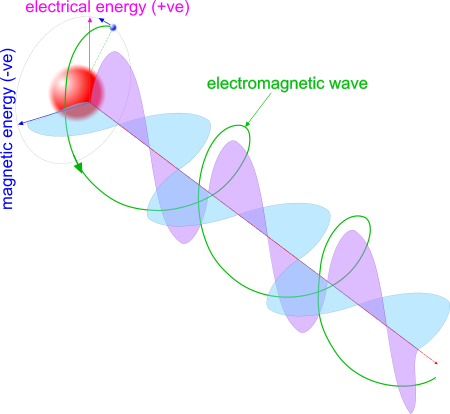
Fig 2. EME Radiation
Very little matter is able to absorb long-wave, low-energy electro-magnetic radiation, it therefore generally travels furthest in an atmosphere (it tends to reflect off solid matter), and is least harmful to life.
Short-wave, high-energy electro-magnetic energy can be absorbed by most organic and inorganic matter and will therefore be lost much more quickly (readily). It therefore tends to travel only very short distances but is most harmful to life.
Radio waves travel around the earth providing television and radio signals without harming the surrounding environment because all matter has difficulty in absorbing such long wave lengths.
On the other hand, x & γ-waves, will be absorbed by almost any organic and inorganic matter and will therefore be lost to their surrounding environment (incl. an atmosphere) very quickly.
In a vacuum, however, all electro-magnetic energy will travel forever until it encounters matter that will absorb it.
Long-wave matter encountering most matter will generally bounce off it (reflect) but short-wave energy will normally be absorbed.
Electro-Magnetic Energy (radiation)
All of the above spectra, from gamma to radio, is how we perceive electro-magnetic [heat & light] energy (EME).
It is radiated by proton-electron pairs and looks like the image in Fig 2
Its properties (ƒ, λ, A, e') at any given temperature can be calculated from the properties of the proton-electron pairs radiating it.
Electro-magnetic radiation is deflected as it passes a large body e.g. a planet or star, due to the potential energy generated by the magnetic charge in all the quanta in the planet or star, i.e. what we currently refer to as gravity.
But you cannot use Isaac Newton's force law to identify this deflection as the EME possesses no mass, you can only use his gravitational constant (G).

Fig 3. Electro-Magnetic Energy
EME (Energy)
There are two principal means of measuring EME energy; Boltzmann and SHC, but they appear to generate different temperatures, for example, a proton-electron pair @ 300K;
Boltzmann: EB = kB.Y.Ṯ = 3.94042969432577E-20 J
SHC: ES = SHC.Y.mₘ.Ṯ = 1.97021484716289E-20 J
EB = 2.ES!
However, EME has two measurable energies; magnitude (KE) and range (PE) (Fig 3).
Magnitude is a measure of the kinetic energy in the orbiting electron generating the EME, and is used in the calculation of specific heat capacity;
SHC = J / K.kg
where 'J' refers to EME magnitude.
Range is a measure of the total energy in EME, and is that defined by Boltzmann in his calculation;
kB = J/K
where 'J' refers to potential energy# in the proton-electron pair generating the EME.
When quoting EME energy, it is usual to specify its magnitude, rather than its range, but they both equally apply.
Therefore, both calculations are correct because they give the same temperature:
Ṯ = KE / SHC.Y.mₘ = PE / Y.kB = 300 K.
# PE = 2.KE in circular orbits such as this in proton-electron pairs
Further Reading
You will find further reading on this subject in reference publications(68 & 70)