Cathodic Protection - DnV RP-B401
(aluminium & zinc anodes)
Cathodic protection is similar in concept to the wet-cell battery and represents a beneficial application for the process of Galvanic Corrosion. See CalQlata’s Nobility calculator.
Cathodic protection is used to preserve the integrity of an important metal body (the cathode) submerged in a corrosive liquid (electrolyte; usually seawater) by using a sacrificial metal (the anode). The protection is effected by the replacement of protons lost from the cathode to the seawater by electrical transfer from the anode. Over time, the anode will become depleted, by losing mass, until it no longer protects the cathode, at which time the anodes will need to be replaced or the cathode will begin to corrode.
Cathodic protection is applicable to both coated and bare-metal structures such as offshore platforms, piers, pipelines, ships, etc.
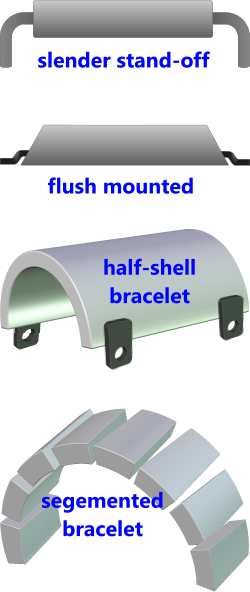
Fig 1. Typical Anode Designs
Anodes
Anodes used for the protection of carbon steel are usullay made from Aluminium or Zinc. Aluminium is preferred for its better electrical properties and lighter weight, but it is less resistant to high tempertures.
There are a number of anode designs that can be used for this purpose as shown in Fig 1. Anode type is normally selected according to the application.
For example:
The slender stand-off unit provides the highest current output but is unsuitable for structural members sensitive to loading or drag (i.e. thin or weak structural members)
The flush-mounted unit is best suited to ships as it has a low drag coefficient. However, the fact that it is so close to the cathode (touching) means that there is a greater risk of localised hydrogen embrittlement (see Hydrogen Embrittlement below).
Bracelets are normally installed on tubular members, such as pipelines, and are easily replaceable as they are attached using bolts and the non-structural welded connections are only for electrical continuity.
Whilst half-shell bracelets will fit only one diameter, they provide a larger protective surface area than the segment bracelet and therefore a higher current output.
The segmented bracelet is made up of numerous flat bars attached to each other by corrosion-proof straps. They can be cut to length and installed on a tubular member of any diameter. They are cheap to install and replace but provide the lowest current output of the bracelets.
Hydrogen Embrittlement (RP-B401 §5.5)
Hydrogen embrittlement is the term used to describe hardening (and cracking) generated in the surface of the cathode material as a result of excessive deposition of single protons (hydrogen ions) that have been electrically detached from the anode.
As anode positioning is fixed, its distance to a protected area will vary. The area closest to the anode will naturally receive a greater deposition of protons than those areas further away. If deposition is occurring faster than the rate of corrosion, this will cause the cathode to become brittle.
Anodes are welded to the protected body generating localised hardening of the steel in the heat affected zone. As these weld-sites are very close to the anode, they are most susceptible to hydrogen embrittlement. The best solution to this problem is to provide properly prepared and applied epoxy coating over the welded area. The alternative to which is heat-treatment of each weld which is both difficult and expensive on large structures.
Refer to RP-B401 (1993), §10.4.5 & B401 (2010), §5.5.11 for recommended action that should be taken for the areas immediately adjacent to anodes.
DnV RP B401 (October 2010)
Note: All paragraph and table references relate to DnV RP-B401
CathPro is based upon the recommendations of the most widely known and accepted recommended practice for this application, which is published by DnV.
This publication includes many general recommendations not included in this calculator such as design, testing and maintenance procedures (see Further Reading below).
Excluded: CathPro does not include the following options in its calculations…
The optional calculation formula for cathodic protection design life that exceeds the life of the coating {§6.4.5}
The factor ‘B’ that may be applied to steel reinforcement in concrete structures with a higher than 5:1 ratio between reinforcement surface area and concrete volume {§6.3.13}
The formula provided for the variation in electrochemical efficiency of aluminium with temperature has been excluded as there is no such calculation for zinc {Fig 6.6.1} (see DnV RP B401 Constants below)
There is no provision for the 5A drain for well casings {§6.9.3}
There is no provision for the drain of 0.0005A/m due to steel armouring of flexible pipes {§6.9.3} as B401 assumes the use of Steel-Non-Bonded flexible pipes which is not always the case. It is recommended that the respective manufacture of HPHT flexible pipe be consulted for actual data.
Included: CathPro has pre-set the following in its calculations…
Initial coating breakdown factor (fcᵢ) is calculated using the same formula as that used for the final value (fcf) but tf is set to zero (i.e. fcᵢ = a) {§6.4.2}
A segmented bracelet is assumed to be made from 8 segments
Thermally sprayed aluminium coating (coat = 4) is given the same breakdown value as coating number III {Table 10.4}
In the cathodic protection calculator, if coat = 0 and depth = 0 all current densities (icᵢ, icₐ and icf) will default to 0.2A/m² {§6.9.2}
The coating breakdown factors for pipelines according to §6.5.3 of the 1993 edition has been retained in CathPro as CalQlata consider this calculation to be relevant and appropriate to the protection of pipelines (ref. §1.2.5).
Cautionary Notes
An increase in design current density of 0.001/°C has been applied in the calculations despite the fact that Tables 10.1 and 10.2 clearly show that current density decreases with environmental temperature {§6.3.11}. If you wish to reverse this modification; i.e. you wish to decrease current density with increased temperature, you should alter the relevant constant in the Data File from 0.001 to -0.001 (see DnV RP B401 constants, "Current density variation for high temperatures {§6.3.9}" below)
You may notice anomalies in the initial and final resistances for slender stand-off and flush-mounted anodes as length to radius ratios close to 4 may result in different formulas being applied to initial and final calculations {Table 10.7}
By setting the water depth to zero, CathPro will assume that the cathode is buried in saturated sediment (e.g. the seabed).
Resistivity is calculated and therefore may vary (very slightly) from Fig 10.1
Calculating the Surface Area for Protection
Any and all steel surfaces that are electrically connected to the body you are protecting will act as a drain on the anode. It is therefore important to include all such areas (A) in your calculation.
It is considered by some to be normal practice to install anodes 96 metres apart⁽¹⁾, or every 8 field joints, for a subsea pipeline. Therefore, it can be assumed that in open water (not buried in sediment) the radial effectiveness of an anode is about 48m. This distance is based upon a bracelet anode and will be dependent upon its mass.
When calculating the number of anodes required for an area to be protected it is normal to break the total area up into smaller areas of different constructions. For example; an offshore steel jacket is generally made up of piles, flat surfaces, tubular members and complex nodes, each of which should be treated individually.
For example;
Piles and skirts can be treated as buried uncoated steel (i.e. depth = 0). Calculate the area of each pile (including guidance structure) or skirt and establish the number of flush-mounted or slender stand-off anodes for their protection. Large anodes can be successfully used for these areas.
Flat surfaces on the seabed can be treated as a single complete surface area on which large slender stand-offs can be attached.
Each tubular member can be fitted with a single central segmented bracelet given that its length of effectiveness is well within the dimensions of an offshore Jacket.
Complex nodes need careful attention as hydrogen embrittlement is a serious issue given the amount of welding. It is usually preferable to install a large number of small anodes strategically located rather than a few large anodes.
Cathodic Protection Calculator – Technical Help
All units are fixed according to RP-B401
You will notice when first installing the cathodic protection calculator or reseting the default data that the calculation produces an error in that final demand is greater than final output. This has been done deliberately in order that you can see how to find a solution. For example; either you can alter the material to zinc or you can increase the design life (which increases the number of anodes required).
Tip: If you find: ‘Final Output < Final Demand’, try reducing the mass (m) of your anodes.
CalQlata’s cathodic protection calculator is a basic calculation facility according to DnV’s Recommended Practice B401. It is not an all-encompassing calculator for every eventuality. Nor does it include recommendations or findings from other publications.
CalQlata fully accept that each user may have individual preferences and recommendations that do not necessarily correspond with those of DnV. For this reason, CalQlata has included the constants recommended by DnV in the 'Data File' in order that you may modify them should you wish to do so.
Should you make changes to the values in this Data File, however, they will be lost if your Data File becomes corrupted and default (original DnV) values will be added to the automatically generated replacement Data File. It is therefore recommended that if you make changes to the Data File, you make a backup copy of the file and save it to disk under a suitable filename. See How They Work for the location of your Data File and how to use it.
Important Note: Take care that you don’t lose a comma separated value in the Data File as all subsequent values in the calculation procedures will be incorrect.
DnV RP B401 Constants
If you open CathPro's Data File, you find the following information provided below the Output Data. You are free to alter any of these values as you wish and the revised values will be used in all future calculations. Should you wish to revert to the originally saved values, simply open the calculator and Reset to Default Data via the ‘File’ menu. When you save the data (menu: File>Save) or exit the calculator a new (replacement) Data File with the 'Default Data' will be saved to disk.
Current density variation for high temperatures {§6.3.9}
25,0.001
Current densities for steel [Tables 10.1 & 2]:
{≤30m, <100m, <300m, >300m}
{initial, average, final}
the four values in each group are for: tropical, sub-tropical, temperate, arctic
0.15,0.17,0.2,0.25,0.07,0.08,0.1,0.12,0.1,0.11,0.13,0.17
0.12,0.14,0.17,0.2,0.06,0.07,0.08,0.1,0.08,0.09,0.11,0.13
0.14,0.16,0.19,0.22,0.07,0.08,0.09,0.11,0.09,0.11,0.14,0.17
0.18,0.2,0.22,0.22,0.09,0.1,0.11,0.11,0.13,0.15,0.17,0.17
Current densities for reinforced concrete [Table 10.3]: {≤30m, <100m, >100m}
the four values in each group are for: tropical, sub-tropical, temperate, arctic
0.0025,0.0015,0.001,0.0008,0.002,0.001,0.0008,0.0006,0.001,0.0008,0.0006,0.0006
Current density for thermally sprayed aluminium {§6.3.11}
0.01
Coating breakdown constants (a & b) {Table 10.4}
0.1,0.05,0.02,0.1,0.025,0.12,0.1,0.05,0.02,0.05,0.015,0.008
Electrochemical Efficiency {Table 10.6}
2000,1500,780,700
Anode Potential {Table 10.6}
-1.05,-0.95,-0.8,-1,-0.95,-0.9
Protective Potential {§5.4 & §7.8.2 Note}
-0.8,-0.9,-0.8
Anode utilisation factor {Table 10.8}
0.9,0.85,0.85,0.8
You may alter any of the above constants but take care not to lose a separating comma.
Accuracy and Applicability
This calculator is as accurate and applicable to the cathodic protection of steelwork submerged in seawater or saturated sediment as the recommended practice on which it is based.
Notes
- Reference publication 40, Chapter 10, §Introduction
Further Reading
You will find further reading on this subject in reference publications(38, 39, 40 & 41)

