Atomic Elements:
Interactive periodic table of atomic elements
Elements is an interactive database and active periodic table of nature's atomic elements.
Periodic Table of Atomic Elements (active)
In the 1860's Dimitri Mendeleev created the periodic table of the elements according to their properties, many of which were missing (unknown) during his lifetime. He recommended that all future chemists concentrate on filling in the gaps. The periodic table is now virtually complete according to their atomic states (see 'Strings' below).
Elements includes an active periodic table (Fig 1), which is accessed by the 'image switch' (CalQlata Logo) at the top of the program window (see How They Work). It displays all the 'Sort Options:' properties (where available) for the elements in the database (1 to 101). Click on any box in the Table to select the element and list its properties in the 'Data Listing' window.
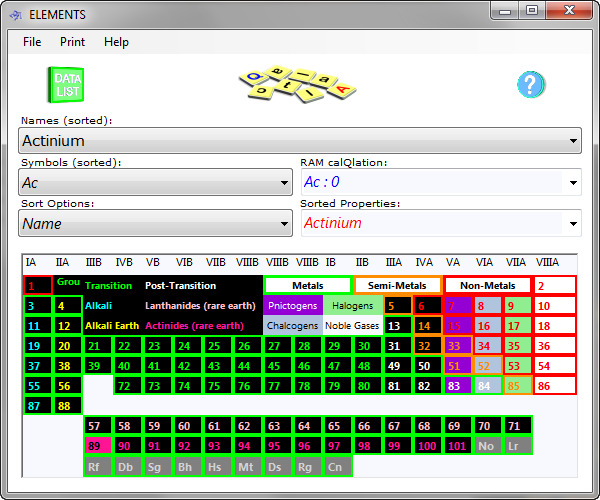
You can select any property box that grabs your interest and Elements will reset the selected element to the one you grabbed. Click on the Data Listing logo at the top of the page and Elements will display all the properties of the newly selected element. You return to the periodic table by clicking on the image switch once again.
The Atom
An atom is a general term used to describe a nucleus (comprising protons and neutrons) surrounded by electrons.
An Element (definition)
An element is defined by the number of protons in its nucleus, which is also known as the atomic number of the atom.
Earth-Bound Properties
Of the 92 naturally occurring elements;
Ten exist naturally on earth as gases (six of which are noble gases)
Two exist naturally on earth as liquids, and
All the others exist on earth naturally as solids
There are eleven non-metals, ten semi-metals and all the rest are metals
Sub-Atomic Particles
About 250 years ago, an 'element' was understood to be nature's smallest component. The atom was defined during the 19th century (e.g. Dalton to Thompson). This too was broken down at the beginning of the 20th century (Rutherford) into a nucleus of protons and neutrons surrounded by electrons.
It is generally accepted today that these are made from even smaller particles: Quarks (Up & Down) and Leptons, and that there are a number of other sub-atomic particles such as Bosons, Gluons, Photons, Gravitons, Neutrinos, Muons, Tauons, Mesons, Baryons, etc. On top of which we now have; dark mass, dark energy, x-rays, gamma-rays, alpha and beta particles, etc. (see also Sub-atomic Physics).
However, sub-atomic particles have recently been shown to be a figment of our imagination; refer to the various science pages dedicated to the elements in our "Useful Stuff" menu, with particular reference to Physics Discoveries, The proton-Electron Pair and The Atom. The smallest (and only) atomic particles in the universe now appear to be the Proton and the Electron. The Neutron, which can only exist inside and atom's nucleus, is a proton-electron pair united through high temperature.
Strings
It is today believed that all these sub-atomic particles may be 'Strings' at different energy levels, that define their individual properties. That is, a Gluon might be a string at energy level '-1', a photon might be a string at energy level '-20, a quark might be a string at energy level '-3', etc. In fact, what we understand today as mass and gravity may not actually exist. It may be that these conditions are simply definitions of a given energy level.
However, as protons and electrons are most probably the only atomic particles in the universe, both of which are definitive and cannot therefore be sub-divided, Strings are most probably also non-existent.
Unification Theory
Given that Relativity and Quantum Theory have at last been discredited, and a Newtonian atom has been conclusively demonstrated, it is now highly improbable that a unification theory will ever be discovered or found necessary to unite atomic and astronomic physics.
Isotopes
An isotope is defined by the number of neutrons in its nucleus with respect to the number of protons (-2, -1, 0, +1, +2, +3, etc.). The number of protons together with the number of naturally occurring neutrons is the relative atomic mass of an element (RAM).
Ions
All materials contain atoms with missing electrons (+ve ion) or excess electrons (-ve ion) and it is their tendency to correct the situation (collect or shed electrons) that results in chemical reactions.
As a general rule (albeit with a few exceptions), liquids and oxygen tend to exist naturally as –ve ions and solids (metals) tend to exist naturally as +ve ions, which is why most metals will oxidise and why most liquids will pass current.
Oxidisation Nºs are the number of electrons that must be added (+) or removed (-) to create the theoretically perfect element.
Atomic Radius is the radius of the principal (most numerous) naturally occurring element including its electrons.
Ionisation Energy 1 is the energy required to remove the first electron from the atom and Ionisation Energy 2 is the energy required to remove the second electron, etc.
Relative Atomic Mass (RAM)
Atomic elements do not exist in nature in their purest form (except for the noble gases). For example, not all atoms in a lump of iron contain 26 protons, 26 neutrons and 26 electrons. Take a look at the RAM values of every atom, none is an exact multiple of its atomic number. If they were, there would be no such thing as a chemical reaction because all atoms would be stable, and life, the universe and everything occurs only because there are chemical reactions.
A chemical reaction is simply two or more elements in close proximity trying to rectify this particle imbalance (see Ions and Isotopes above).
Electronegativity
An atom's electronegativity level (its tendency to collect electrons) will give you some idea of its potential for chemical reaction.
Whilst it is not a 'rule', you tend to find that the higher a metal's electronegativity, the more noble (stable) it will be and the less its tendency to corrode or chemically react.
The lower its electronegativity (the more electropositive) the more likely it will be to corrode or chemically react.
If you bond two atoms together with the same electronegativities, they will form a covalent bond and will be electrically neutral. As the electronegative difference between bonding atoms increases the resultant molecule will become more 'polar' (one end positive and the other end negative, so to speak). For example: the positive acid & the negative alkaline ions join together to make polar (but pH neutral) water, a molecule with one positive node and one negative node making water such a good electrolyte.
Electronegativity increases from the bottom-left corner of the periodic table to the top-right hand corner (excluding the smallest noble gases {Helium, Argon & Neon} which tend to be neutral – they don't bond).
Electron Shells
These are the orbital paths followed by the electron partners about their proton partners in an atomic nucleus. They are not elliptical, nor do they comprise sub-shells (s, p, d, f,) as we have been told today. Each shell (which is circular) contains two electrons - except the outermost shell, which can contain one or two electrons. They are described True Atom. There are no valences, when an inner electron is knock from its orbit, all the outer electrons will relocate inwards to ensure electrical balance.
Fission & Fusion
Fission is the splitting of nucleic neutrons into their component parts and thereby releasing their stored energy. This occurs naturally as a result of atomic decay and unnaturally in stars as a result of internal heat generated through spin.
Fusion is the forcing of the nucleus of one atom inside the electron shells of another. This process does not release energy, it requires the input of energy to occur. That energy comes from gravitational force in the core of the universe's most massive bodies; galactic force-centres, the great attractor and the ultimate body.
Radioactive Decay
Radioactive decay occurs over time as atoms gradually release unwanted neutrons - splitting them into their component parts; a proton and an electron - in an attempt to achieve their most stable condition; a neutronic ratio of 1:1.
Table of Elements - Technical Help
Data Accuracy
CalQlata would like to raise a word of caution concerning the property values included in this database.
The data contained in this calculator has not come from physical and/or chemical tests carried out by CalQlata. We have collected this data over a long period from sources we believe to be reliable. CalQlata's reliable sources tend to be published documents, reference books and test-houses. Where possible, we have tried to corroborate the accuracy of this data with at least two additional sources we also believe to be reliable. Where this has not been possible, we have used internet sources for a tertiary check. However, as we have no way of knowing the quality assurance levels of internet websites, we have added the cautionary note '{?}' to such values.
One particularly difficult property to nail down reliably is the boiling point of Silicon, which varies considerably, even between reliable sources (2588K to 3540K). After much deliberation and research, we concluded that the most reliable value appears to be 2873K!
Almost all of the data you will find on the elements will be approximate, irrespective of its source. It is not, for example, possible to define accurately the melting temperature of a material unless you can guarantee its 100% purity along with similar accuracy levels of the test conditions, neither of which are representative of 'real-world' conditions and is not the case for most tests performed on elements to date. Furthermore, heat treatment or physical working can modify the performance characteristics of some metals. This is why specified minimum yield stress (SMYS) and ultimate tensile stress (UTS) are sometimes specified as a range.
We have calculated some of our data where it has been difficult to locate and corroborate using reliable sources but where an accurate or recognised formula is available. For example:
1) The calculation of elastic properties is as accurate as the data used in the calculation (with no error margin).
2) Where latent heat information has been difficult to locate using reliable sources, the missing values have been established using the 'Chen' formula(12), which is accurate to between 2% and 6%.
Although we believe that data calculated using reliable variables is more dependable than data from sources unknown to us we qualify such data with the extension: '{Q}'.
If your work requires the use of accurate information, it is always preferable to perform your own tests or at least acquire the information from a recognised test-house.
You will find further reading on this subject in reference publications(12 & 15)

